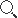Non-pneumatic Anti-shock Garment(NASG), size S/M/L
Non-pneumatic anti-shock garment (NASG), size small/medium/large. First aid device used in the management of postpartum haemorrhage (PPH) to stabilize women in hypovolemic shock till further treatment is available.
DESCRIPTION:
A small/medium/large sized, non-pneumatic anti-shock garment (NASG) to be used for managing uncontrollable postpartum haemorrhage (PPH) and to keep women alive until they can get the treatment they need.
GENERAL DESCRIPTION:
Lightweight, flexible and comfortable for the wearer.
Reusable.
No metal parts: safe for X-rays and MRIs.
Not use of PVC polymer.
Free from hazardous chemicals, shall not cause irritation and allergy to users.
Material: Made of a lightweight neoprene garment and Velcro.
The NASG must allow perineal access so that examinations and vaginal procedures can be performed without it being removed.
Shape:
- Made in the shape of trousers divided into five or six segments for ease of application to different parts of lower body below the diaphragm with Velcro fasteners.
- The segment 1 must consist of 2 pieces to be used in each ankles ,
- The segment 2 must consist of 2 pieces to be used in each calf,
- The segment 3 must consist of 2 pieces to be used in each thigh,
- The segment 4 must consist of 1 piece to be used around the pelvis ,
- The segment 5 and/or 6 must consist of 1 piece to be used aground the abdomen/umbilicus. This segment must apply extra compression with a foam ball.
- The garment must be able to apply 30 to 50 mmHg of pressure to the lower body pressure.
- Life time: At least 200 uses(Washing with brush has a lifespan 174 uses).
Suitable for washingmachines.
INSTRUCTIONS FOR USE:
Intended for women suffering from uncontrollable postpartum haemorrhage (PPH), to control the bleeding, reverse the shock, and stabilize the patient for safe transport to a comprehensive obstetric care facility.
The NASG applies pressure to the lower body and abdomen, thereby stabilizing vital signs and resolving hypovolemic shock.
When fitted correctly, the NASG reverses the shock by returning blood to the essential organs - heart, lungs, and brain.
1. Place the NASG under the woman;
2. Close the segments 1 tightly around the ankles;
3. Close the segments 2 tightly around each calf;
4. Close the segments 3 tightly around each thigh, leave knees free;
5. Close the segment 4 around pelvis with lower edge at level of pubic bone;
6. Close the segment 5 with pressure ball over the umbilicus and finally, close the NASG with segment 6;
The segments 1, 2, 3 can be applied by two persons simultaneously, but segments 4, 5 and 6 should only be applied by one.
Make sure the woman can breathe normally after close the NASG.
It is extremely important not to remove the NASG before a woman receives IV fluids, blood and before all vital signs are restored. Early removal can be dangerous and even fatal.
For cleaning: Mix a 0,01% bleach solution, immerse the NASG in that solution and soak for 10min. Scrub to remove tissue and particles. Wash and rinse with clean and cool water. Squeeze and hang in the sun to dry it.
SUPPLIED WITH:
Manufacture's instructions for use in Chinese, English, French and Spanish.
PACKAGING AND LABELLING:
One small sized NASG, packaged individually with full instructions in English, French and Spanish.
Labelling on the primary packaging:
- Name and/or trade mark, and address of the manufacturer.
- Manufacturer's product reference.
- Type of product and main characteristics.
- information for product-specific storage conditions (e.g., temperature, pressure, light, humidity, etc.).
SAFETY & PRODUCT STANDARDS(Comply with following standards):
- ISO 13485:2016 Medical devices -Quality management systems -- Requirements for regulatory purposes
- ISO 14971:2019 Medical Devices- Application of risk management to medical devices
- ISO 10993-1:2020 Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process
- ISO 10993-5:2009 Tests for in vitro cytotoxicity
- ISO 10993-10:2021 Tests for skin sensitization
- ISO 13934-1:2013 Determination of maximum force and elongation at maximum force using the strip method
- ISO 6330:2021 Tensile strength loss after washing
Regulatory Approval:CE